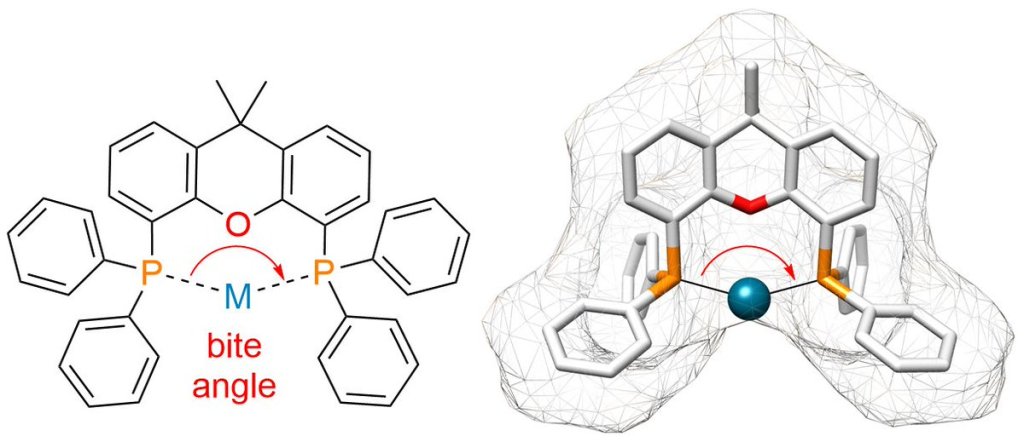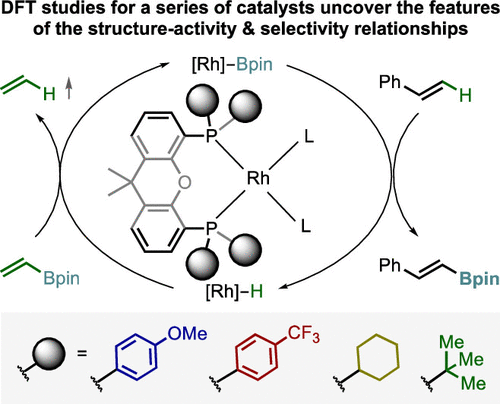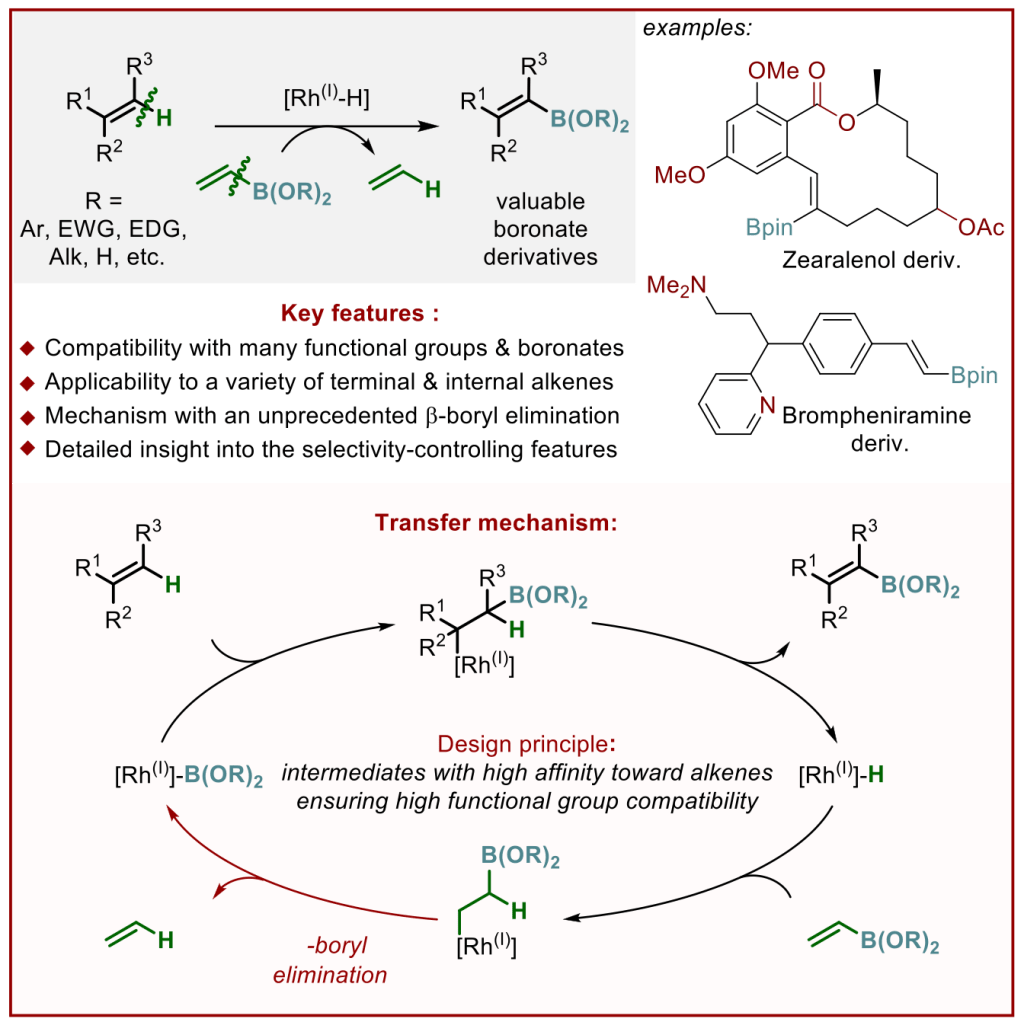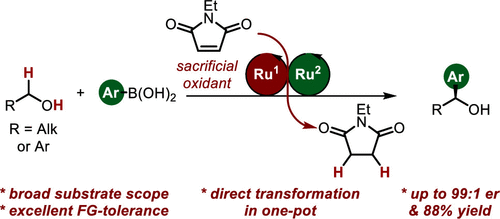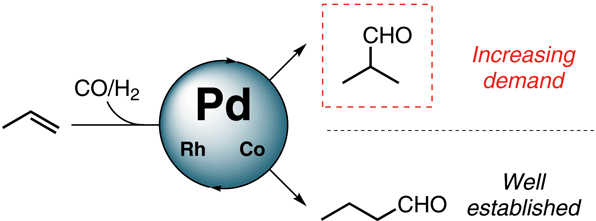
The hydroformylation of simple aliphatic alkenes, such as propylene, is one of the largest homogenous catalyzed processes in the chemical industry, producing over 10 million metric tons of different aldehydes each year. Typically, such processes are catalyzed by Co or Rh catalysts, yielding mostly linear aldehydes, such as n-butanal. However, the increasing demand for branched aldehydes, such as isobutanal, triggered further investigation to develop efficient isoselective protocols, which remain scarce. In this Synpacts article, we discuss our recent work on iodide-assisted Pd catalysis as an attractive alternative strategy for the development of isoselective methods. This article is presented considering the state of the art for Rh-catalyzed processes. Additionally, we discuss the limitations and challenges that need to be addressed in order to successfully transfer the technology to industry.
Synlett 2023, 34, 1185–1194.
Congrats to all!