
Yifei Bai joins the group as a PhD student. Warm welcome to Yifei!


Yifei Bai joins the group as a PhD student. Warm welcome to Yifei!

We’re very happy to welcome several new members to the group this term: PhD students Nicolás Manno, Daniel Read, and Oscar Hills; MPhil student Qihao Huang; Part III student Dana Moss; and visiting MSc students Melvin Bezjak (RWTH Aachen University, Germany) and Marie Carlier (École Normale Supérieure Paris-Saclay, France).
All make an appearance in our long-overdue new group photo above — it’s been a while!
We’re thrilled to share that Kuhali has been awarded a Poster Prize at the 28th International Symposium: Synthesis in Organic Chemistry! Her poster, “Versatile dehomologative functionalizations of aldehydes and alcohols enabled via Rh-catalyzed multi-auto-relay”, highlights work recently published in J. Am. Chem. Soc. 2025, 147, 16735.
A huge bravo to Kuhali for this recognition!

Gracjan Kurpik joins the group as a postdoctoral researcher. Warm welcome to Gracjan!

Our latest study introduces a rhodium-catalyzed one-pot method that converts aldehydes and alcohols into vinyl and alkyl boronates via sequential transfer dehydroformylation, isomerization, and borylation. This multicatalytic approach streamlines C–C bond functionalization and expands access to valuable organoboronates from common starting materials. Beyond its synthetic utility, the study showcases the power of multicatalysis—multiple catalytic events using a single catalyst system—to simplify complex transformations and improve the efficiency of fine chemical synthesis.
Read the full paper here.
We are excited to offer a fully funded PhD position for UK-based candidates (home fees) in our research group! This is a fantastic opportunity to work at the cutting edge of catalysis and organic synthesis in a dynamic environment.
🔬 What we’re looking for:
✔️ Passionate and motivated candidates
✔️ Strong academic background in chemistry
✔️ Interest in our research—tell us what excites you!
📩 How to apply:
Send your CV, university transcript, and a cover letter to pd552@cam.ac.uk. In your cover letter, let us know which area of our research interests you the most!
Don’t miss this chance—apply now and take the next step in your scientific career! 🚀
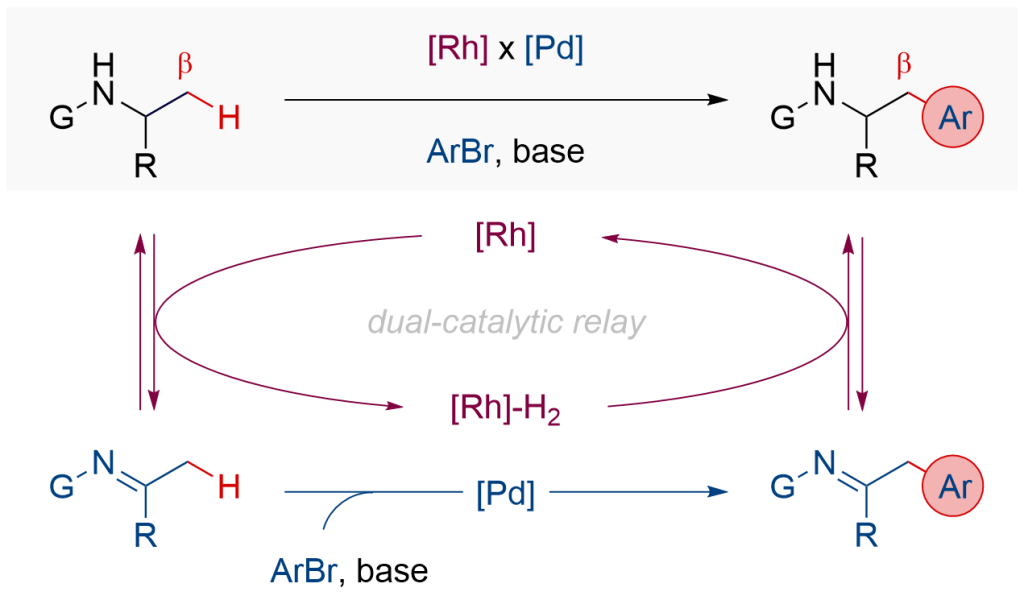
In this paper, we report our studies of a dual relay catalytic protocol for regioselective arylation of amines at their unactivated β-C(sp3 )-H bond. The strategy merges reversible dehydrogenation of amines by Rh catalysis and C-H functionalisation of transient imines by Pd catalysis, enabling the reactions for previously inaccessible starting materials. In a broader sense, this study demonstrates new opportunities in dual relay catalysis involving hydrogen borrowing chemistry, previously explored in the functionalisation of alcohols, to execute otherwise challenging transformations for amines, commonly present in natural products, pharmaceuticals, biologically active molecules, and functional materials.
https://pubs.rsc.org/en/content/articlepdf/2025/SC/D4SC06806H?page=search
Congrats to all!

Daniel – Part III Student

Pawel – Part III Student

Robert – PhD Student

After an excellent presentation and a thorough scientific discussion with the jury members – Dr. Laurence MIESCH, Prof. Zheng-Hui GUAN, and Prof. Matthias BELLER, Michel successfully defended his PhD thesis and was granted a doctorate title. Congratulations, and good luck for the future! Special thanks to the jury members!

After an excellent presentation and a thorough scientific discussion with the jury members – Prof. Marine DESAGE-EL MURR, Prof. Chao WANG, Prof. Timothy DONOHOE, and Prof. Jacek MLYNARSKI, Bruno successfully defended his PhD thesis and was granted a doctorate title on 20/12/2023. Congratulations and good luck for the future! Special thanks to the jury members!