
Jan Bojanowski joins the group as a postdoctoral researcher. Welcome Jan!


Jan Bojanowski joins the group as a postdoctoral researcher. Welcome Jan!
After an excellent presentation and a thorough scientific discussion with the jury members – Prof. Martin Oestreich, Dr. Adrien Quintard, Dr. Stéphane Bellemin-Laponnaz, Lukas successfully defended his PhD thesis and was granted a doctorate title. Congratulations and good luck for the future! Special thanks to the jury members!

Flora Mammadova joins the group as a PhD student. Welcome Flora!

Our recent perspective on ‘Recent Trends in Group 9–Catalyzed C−H Borylation Reactions: Different Strategies to Control Site-, Regio-, and Stereoselectivity.‘ has been selected for the cover of the ‘Bürgenstock Special Issue 2021 – Future Stars in Organic Chemistry’.
https://www.thieme-connect.com/products/ejournals/issue/10.1055/s-012-54341
Congrats to all!
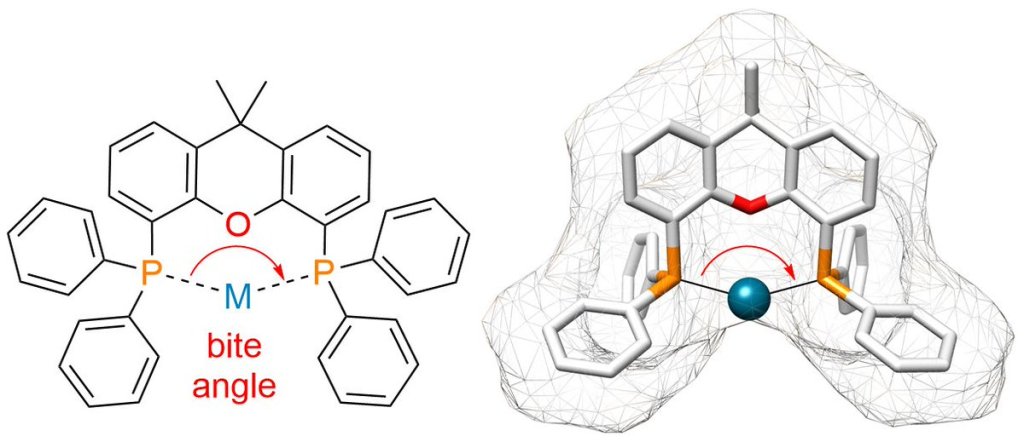
In this comment we discuss the importance of ligands in transition metal catalysis, looking at the success story of xantphos and why we feel it should earn the title of ‘privileged ligand’.
https://www.nature.com/articles/s41557-022-01031-x
Congrats to all!
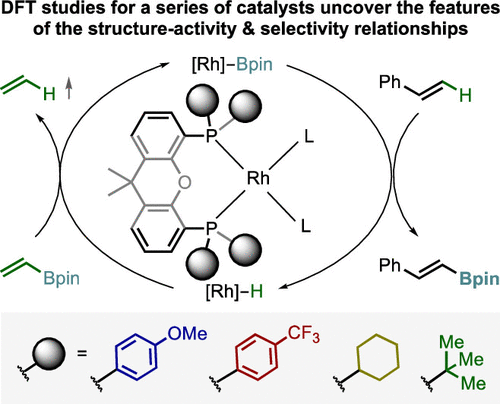
In this paper, just reported in Organometallics, we describe the results of a computational investigation that shed light on the catalyst structure–activity and selectivity relationships for our recently developed Rh(I)-xantphos-catalyzed transfer C–H borylation of alkenes (c.f., Chem Catal. 2022, 2, 762-778). The study provides guidelines for the rational development of new catalysts to further enhance the performance of the catalytic system and address the remaining challenges.
https://pubs.acs.org/doi/10.1021/acs.organomet.2c00148
Congrats to all!

After an excellent presentation and a thorough scientific discussion with the jury members – Prof. Bill Morandi, Prof. Bas de Bruin, Prof. Philippe Dauban, Dr. Joanna Wencel-Delord, and Prof. Thomas Ebbesen, Sebastián successfully defended his PhD thesis and was granted a doctorate title. Congratulations and good luck for the future! Special thanks to the jury members!

Our recent publication on “Transfer C–H borylation of alkenes under Rh(I) catalysis: Insight into the synthetic capacity, mechanism, and selectivity control.” is featured on the cover of the April issue of Chem Catalysis!
https://www.cell.com/chem-catalysis/issue?pii=S2667-1093(21)X0012-7
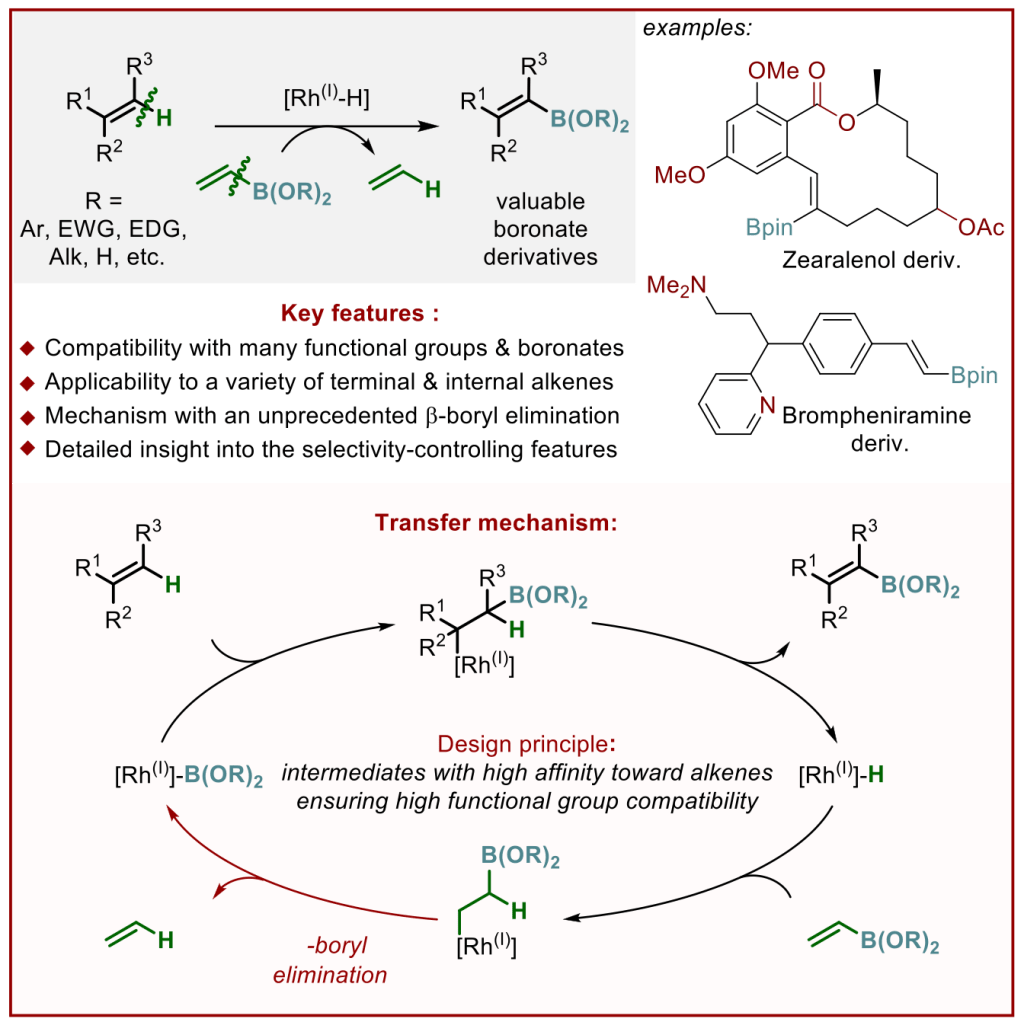
In this paper, we present a broadly applicable method for the C–H borylation of various alkenes, that is, a valuable transformation in the synthesis of fine chemicals, such as pharmaceuticals and agrochemicals. Importantly, the reaction tolerates a plethora of functional groups and can be used for the late-stage functionalization of complex bioactive molecules, such as derivatives of zearalenol and brompheniramine. The study provides insight into the reaction mechanism and the features controlling the selectivity, thereby setting the stage for the development of other related valuable reactions.
https://www.cell.com/chem-catalysis/fulltext/S2667-1093(22)00103-8
Congrats to all!
Hanusch Grab leaves the group to join SpiroChem as a Research Scientist!
Good luck!